Taking a breakthrough from the lab bench to the real world is one of the biggest challenges biotech startups face. The journey is complex — full of scientific hurdles, regulatory checkpoints, and business decisions that can make or break success. Yet, with the right strategy and support, startups can significantly shorten the time between innovation and impact.
At ComingTechs, we’ve spent years guiding biotech startups through this exact journey. Here’s a practical look at how you can accelerate your product launch — without compromising on quality, safety, or compliance.
1. Start with a Clear Product Roadmap
Every successful biotech launch begins with a clear and realistic product roadmap. It’s not just a timeline — it’s a strategic plan that aligns scientific development with regulatory requirements and market needs. Your roadmap should:
- Define key milestones (proof of concept, preclinical, clinical, etc.)
- Identify regulatory requirements at each stage
- Set go-to-market goals and timelines
- Align R&D with business priorities
Too often, startups lose time and funding because they build first and plan later. A roadmap prevents that.
2. Build Regulatory Awareness Early
One of the most common pitfalls? Treating regulatory planning as an afterthought.
Whether you aim for FDA clearance, CE marking, or ISO 13485 certification, regulatory compliance must be built into your product from the beginning.
Steps to take:
- Engage regulatory experts early
- Understand the classification of your product
- Start documentation and QMS setup in parallel with development
- Avoid rework by designing for compliance
At ComingTechs, we help startups align with FDA, ISO, and HIPAA standards right from the design phase — saving months of backtracking later.
3. Secure Funding with Strategic Proposals
Funding is the fuel for your product journey. But scientific merit alone won’t win grants. Whether applying for SBIR, NIH, NSF, or DoD support, your proposal must speak the language of the funders.
Tips to strengthen your application:
- Clearly state the public health impact
- Align with the agency’s current priorities
- Include a commercialization plan
- Show regulatory readiness
Our team has supported hundreds of successful funding proposals that not only got approval but also laid the foundation for market entry.
4. Implement a Scalable Quality Management System (QMS)
As your product advances, so do the expectations for quality and traceability. A QMS aligned with ISO 13485 ensures your processes are consistent, auditable, and scalable.
What a good QMS should include:
- Design controls and change management
- Risk management procedures
- Supplier qualification processes
- Audit and training records
Setting this up early avoids last-minute scrambles before audits or product submissions.
5. Leverage AI and Cloud Infrastructure for Speed and Scale
Modern biotech products often generate and rely on large volumes of data. Whether it’s diagnostic tools, wearable health devices, or AI-powered platforms, your tech needs a robust and compliant cloud infrastructure.
What we recommend:
- Use HIPAA-compliant cloud services
- Implement AI for data processing and decision support
- Automate reporting and analysis for faster iterations
At ComingTechs, we design AI and cloud solutions tailored for healthtech startups — enabling faster clinical insights and better patient outcomes.
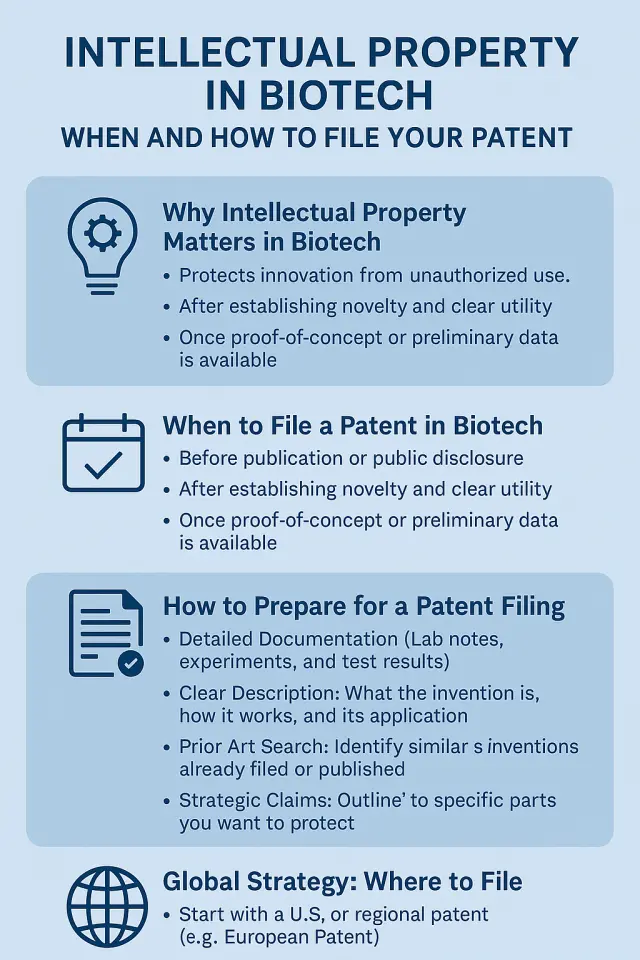
6. Protect Your Innovation with the Right IP Strategy
Speed doesn’t mean skipping protection. Filing patents at the right time, in the right jurisdictions, with a clear scope can protect your invention and attract investors.
Key IP tips:
- File a provisional patent early in development
- Perform a freedom-to-operate analysis
- Align your IP with your go-to-market plan
We provide IP strategy consulting rooted in USPTO expertise and global patent frameworks.
Final Thoughts: Partnering for Acceleration
Launching a biotech product is like navigating a maze. You need the science, but you also need a strategy, compliance know-how, and the ability to move fast without breaking things.
That’s where we come in.
At ComingTechs, our mission is to help innovators bring life-changing solutions to market — faster, safer, and with global impact. Whether you’re building a diagnostic tool, a new therapeutic, or an AI-driven platform, we’re here to guide you from lab to market.
Ready to launch smarter and faster?