Arterys Receives FDA Clearance for Liver AI and Lung AI Lesion Spotting Software
Liver AI and Lung AI Lesion Spotting Software
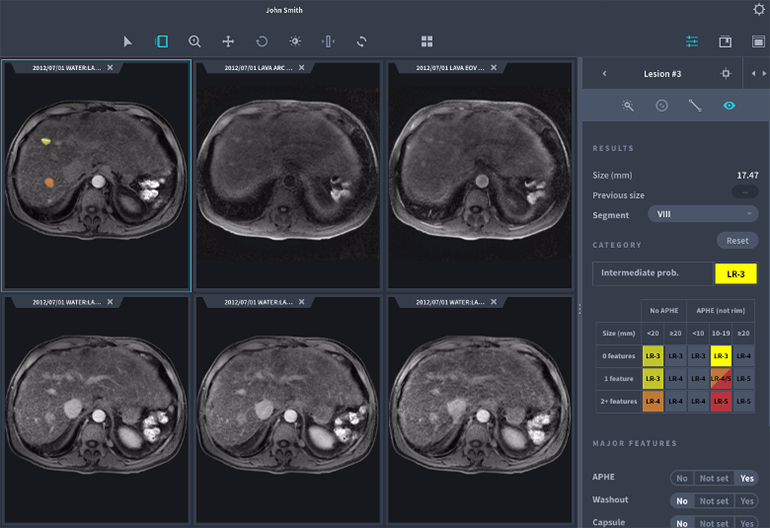
Liver AI and Lung AI Lesion Spotting Software aids in finding lesions within CT images of the lungs (Lung AI) and in both CT and MRI when assessing the liver (Liver AI). It uses artificial intelligence methods to segment lesions and nodules, and in evaluations versus certified radiologists it performed as well as them.
In Feb. 15, 2018 Arterys Inc., the leader in intelligent, cloud-based medical imaging software solutions, received its fifth 510(k) clearance from the U.S. Food and Drug Administration (FDA). The clearance is for the Arterys Oncology AI suite, and is a milestone indicative of the company’s momentum in applying AI to advance medical imaging accuracy and consistency.
Arterys
Arterys was founded in 2011 to facilitate the global advancement of healthcare and enable data driven medicine by leveraging cloud computation and artificial intelligence. Its first major milestone was the first-ever clearance of cloud-based deep learning software for clinical use. Arterys offers a suite of applications for clinicians on the Arterys network via MICA, its web-based AI platform. MICA enables use and interaction with deep learning algorithms in real-time, augmenting the clinician and expediting image interpretation.
News & Image resource:
https://arterys.com/news
https://www.medgadget.com/2018/02/arterys-fda-clearance-liver-ai-lung-ai-lesion-spotting-software.html
Find new and coming technologies at our site: https://comingtechs.com. Please subscribe our news form and add RSS to your favorite news reader.[mc4wp_form id=”153″]


Post Comment
You must be logged in to post a comment.